Clinical Trials

Clinical trials are part of our mission to develop and make innovative medicines available worldwide.
In these clinical trials, which are only possible because volunteers (both healthy and sick) agree to participate, we follow best practice in terms of safety and transparency.
Medicines save and transform people's lives, but the process of developing and approving a medicine is long, rigorous and complex, taking several years to complete. Initially, medicines are studied in a laboratory during the so-called pre-clinical phase. If the initial results are promising, the experimental medicines undergo rigorous testing to determine whether they work effectively, are stable, and produce reproducible and reliable results. These tests can be done using computer simulations, cell culture models, or, only when strictly necessary, on animals. If the medicines pass this stage, they are formulated into safe and robust medicines that can be used in clinical trials, so that they can be carefully tested in people. Clinical trials aim to see if a medicine is safe and effective in treating or preventing the disease for which it has been developed; if it achieves better results than existing therapies; and if it improves patients' quality of life The approval of a medicine by regulatory authorities and its entry into the market depends on the submission of a scientific dossier, of which the results of clinical trials are a fundamental component.
Phases of clinical trials
Clinical trials are carefully designed, reviewed, and programmed, and must be approved by regulatory authorities and ethics committees before they are initiated. People of all ages may participate, according to pre-specified eligibility criteria.
Clinical trials are divided into four phases:
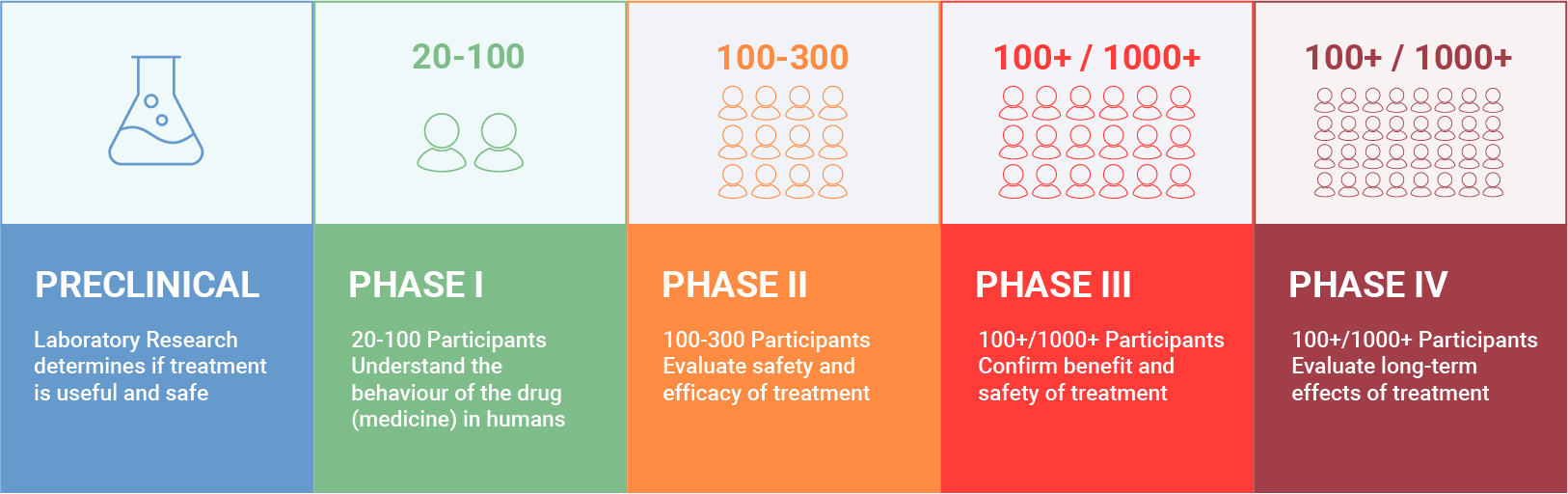
Phase I
20-100 healthy volunteersThe purpose of this phase is to evaluate the medicine's safety and discover any adverse effects. The aim is to understand how the medicine works in the human body, and to determine what is an appropriate dose to use in the phases that follow. This phase is short, and volunteers are monitored very closely at the research centre where the study is being conducted.








